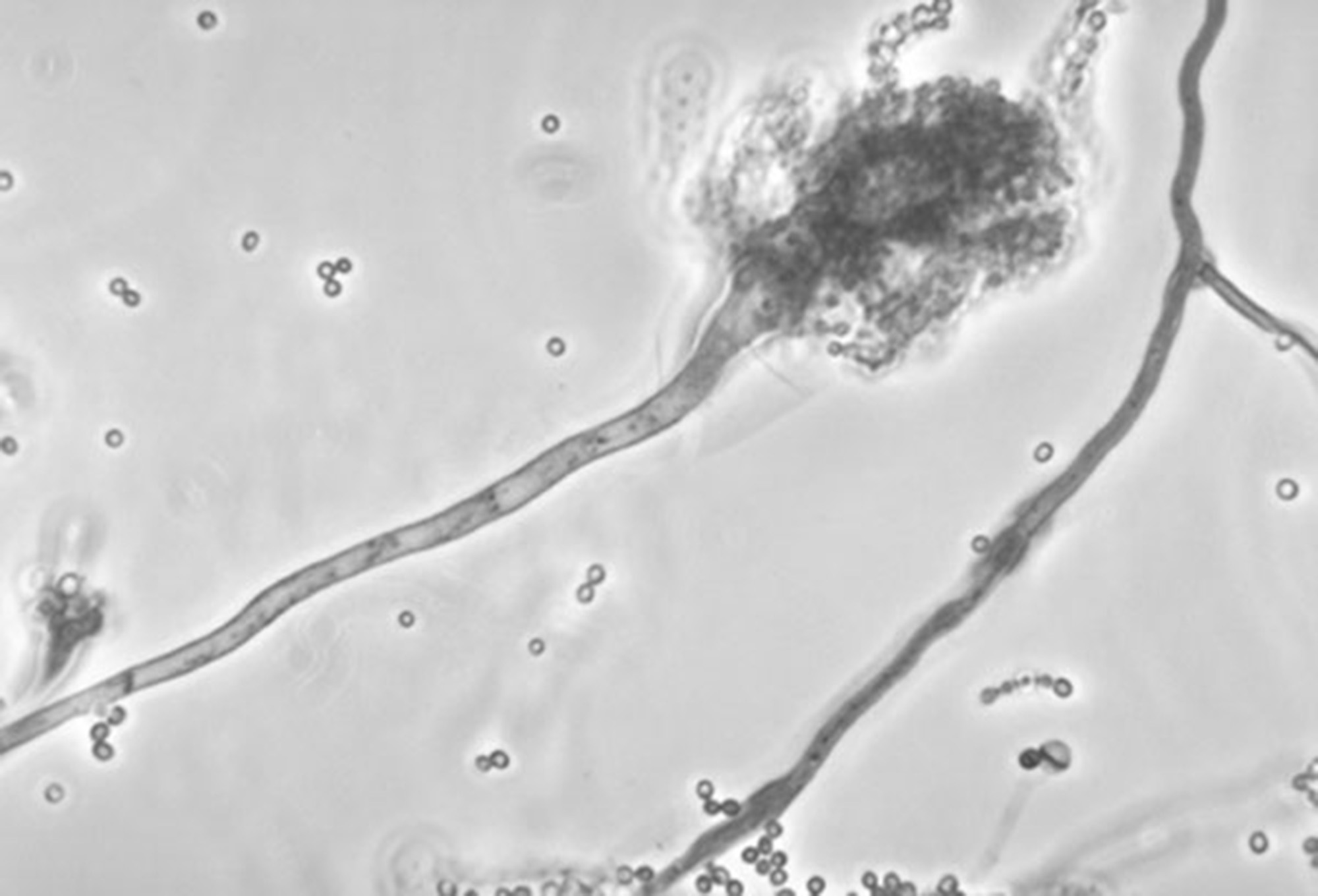
 This undated photo made available by the Centers for Disease Control and Prevention shows a branch of the fungus Aspergillus fumigatus. The fungus can also cause skin infections if it enters a break in the skin. The meningitis outbreak is linked to the fungus being accidentally injected into people as a contaminant in steroid treatments. It's not clear how the fungus got into the medicine.
This undated photo made available by the Centers for Disease Control and Prevention shows a branch of the fungus Aspergillus fumigatus. The fungus can also cause skin infections if it enters a break in the skin. The meningitis outbreak is linked to the fungus being accidentally injected into people as a contaminant in steroid treatments. It's not clear how the fungus got into the medicine.As of Oct. 13, 185 cases of rare fungal meningitis associated with 14 deaths have been reported in 12 states. Strokes were complicating factors in several patients. Epidural corticosteroid injections have been implicated in each case. Tennessee leads the nation with 50 cases and six deaths. Medication suspected of contamination with fungal spores originated in a compounding pharmacy in Boston. An unopened vial from the company was found to be contaminated with the microorganisms.
Injections with possibly contaminated medications were given in 72 clinics during a two-month span beginning July 20. As many as 15,000 patients may have been injected with the contaminated drug.
Fungal meningitis is rare. The two fungal types implicated in the outbreak, Aspergillus and Exserohilum, are exceedingly rare causes. These fungi are commonly found in nature. Ordinarily, they pose risks only to people with impaired immunity or chronic infections.
Because fungal meningitis may develop slowly, new cases related to the suspected contaminated drug may occur for months to come. Corticosteroid could block the body's normal response to fungi invasion.
Epidural injections of corticosteroids are commonly mixed with a fast-acting, local anesthetic agent for administration to patients with chronic lower back pain. An estimated 5 million injections were given in the U.S. in 2011. The injecting needle is carefully positioned inside the bony spinal column but outside the membranes that contain the spinal cord and its surrounding fluid. Patients who respond to injections may experience relief from pain for weeks to months.
The Food and Drug Administration has authority to regulate the manufacture of prescription medications. Companies that compound medications from existing, approved substances register as pharmacies within the state of their location. They are governed by state regulations, which vary widely across the nation.
Compounding pharmacies can customize drugs for individual patients. They may offer substantially cheaper, generic alternatives to pricey, brand-name drugs. Compounding pharmacies have sometimes provided vital drugs in the face of national shortages. Most pharmacies serve a local or regional market. New England Compounding Center, the producer of the suspect corticosteroid, produced large numbers of vials that were shipped to clinics and hospitals in at least 23 states.
Opponents of federal regulations in general argue that the states should assume many of the responsibilities of agencies such as the FDA. The unfolding calamity of drug-related fungal meningitis is a powerful counter argument. Individual states cannot establish and operate sophisticated labs to assure drug safety. They cannot recruit trained professional staff to inspect manufacturing facilities. Another federal agency, the Centers for Disease Control and Prevention, provides unmatched services to identify and track epidemics.
Standards for the manufacture, packaging and distribution of drugs must be uniform across the country. If a compounding pharmacy engages in interstate distribution of its products, it must be subject to the same oversight that applies to traditional pharmaceutical companies.
This disaster also highlights other vulnerabilities in our supply of prescription drugs. Oversight of drugs manufactured abroad is imperfect. Hundreds of Americans were sickened with many deaths in 2010 when China shipped a counterfeit material to the U.S. for use in making heparin, a clot-preventing drug.
Internet sales of drugs pose another threat. More than 4,100 websites offer online prescription drugs, often without requiring a physician's order. Three companies sponsor the great majority of the sites. The websites have been accused of distributing fake, impure and unapproved medications. Some have distributed controlled substances such as narcotics and psychoactive drugs without prescriptions. On Oct. 8, possibly in response to the meningitis outbreak, the FDA ordered the shutdown of these websites.
We take for granted pure drinking water, air free of toxins and safe drugs. There are major holes in the regulations that assure safe drugs. Closure of these gaps is urgent and worthy of strong, bipartisan support.
Email Clif Cleaveland at cleaveland1000@comcast.net.