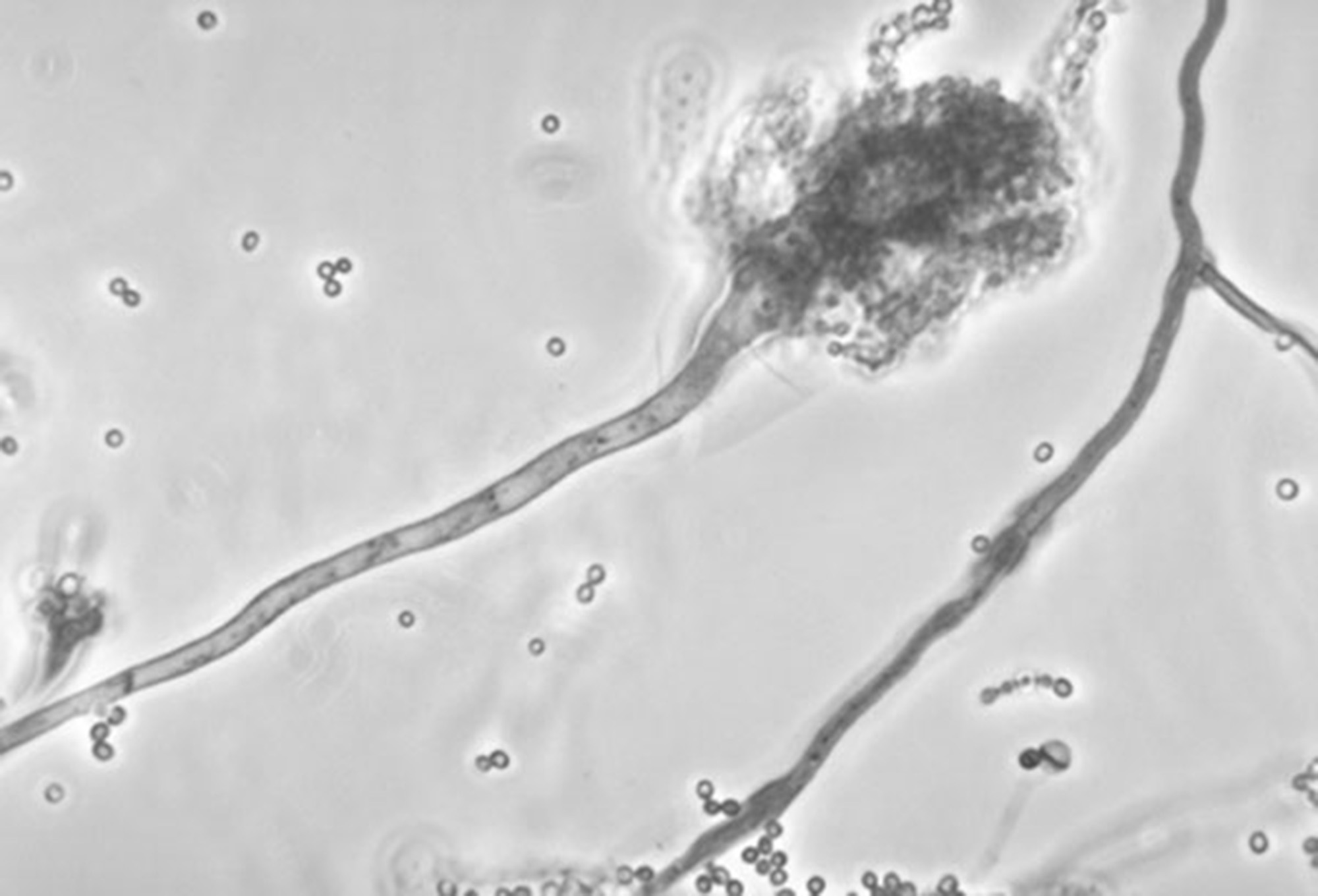
 This undated photo made available by the Centers for Disease Control and Prevention shows a branch of the fungus Aspergillus fumigatus. The fungus can also cause skin infections if it enters a break in the skin. The meningitis outbreak is linked to the fungus being accidentally injected into people as a contaminant in steroid treatments. It's not clear how the fungus got into the medicine.
This undated photo made available by the Centers for Disease Control and Prevention shows a branch of the fungus Aspergillus fumigatus. The fungus can also cause skin infections if it enters a break in the skin. The meningitis outbreak is linked to the fungus being accidentally injected into people as a contaminant in steroid treatments. It's not clear how the fungus got into the medicine.NEW YORK - Four more people have died in the national meningitis outbreak, bringing the death toll to 19, health officials said Wednesday.
The deaths are among the 247 people in 15 states sickened in the outbreak. They all received shots of an apparently contaminated steroid medication made by a Massachusetts specialty pharmacy.
Most of the patients contracted a rare fungal form of meningitis, after getting the shots for back pain over the past few months. Two developed infections from joint injections.
Of the latest deaths, two were in Tennessee and one each was reported in Florida and in Virginia, the Centers for Disease Control and Prevention reported Wednesday. That brings deaths to eight in Tennessee; three in Florida and Michigan, two in Indiana and Virginia, and one in Maryland.
Test results so far show infections with three kinds of fungus, most of them a form of black mold, the CDC said. Of 42 patients, 40 were infected with Exserohilum fungus. The others were infected with Aspergillus or Cladosporium. All are treated with the same anti-fungal medications.
Three lots of the suspect steroid were recalled last month by the New England Compounding Center of Framingham, Mass. All the illnesses have been traced to one of those lots.
Food and Drug Administration officials last week said they found fungus in 50 vials of the preservative-free steroid called methylprednisolone acetate. However, they have not said what kind of fungus they detected.
On Monday, FDA officials said they are investigating two more drugs made by New England Compounding - another steroid and a solution used during heart surgery. Initially, the FDA said two heart transplant patients who got the heart solution developed fungal infections but later said one case involved a solution made by another company. They also have cautioned there could be other explanations for the infections.\